The Best Guide To Copier Solutions In Santa Fe Springs Ca
Table of ContentsWhat Does Document Management In Santa Fe Springs Ca Do?Indicators on Managed It Services In Santa Fe Springs Ca You Need To KnowWhat Does Copier Solutions In Santa Fe Springs Ca Mean?Managed It Services In Santa Fe Springs Ca - The FactsOffice Technology Supplier In Santa Fe Springs Ca for Beginners
Organizations with an on-premises DMS are accountable for their very own protection. This kind of DMS does not depend on the net-- if the web link decreases, the DMS individuals can still access all their files. The drawback of on-premises DMSes is the large ahead of time costs, plus annual expenses for software program updates.A cloud-based DMS is available to the organization online. Users of a cloud-based DMS do not require to back up their files because they immediately save in the cloud.

Cloud-based DMSes depend completely on the supplier to maintain the system up as well as running, while the on-premises system relies on business's own IT resources. A DMS allows businesses to scan, store as well as retrieve company files, but it has added functions that consist of: allows individuals to categorize records with metadata fields; rises findability of web content within the DMS; makes it possible for users to see a picture of the paper without having to mount its additional software application; allows users to modify and also produce new versions of documents; enables users to see all modifications that customers make to a document and also to recover older versions of files; allows users to share records with interior or exterior users; supply customers with the capability to regulate which individuals or teams can access papers and what level of accessibility they have; and enable managers to establish the operations of files throughout an organization.
How Document Management In Santa Fe Springs Ca can Save You Time, Stress, and Money.

A DMS needs customers to visit to the system, which offers an additional layer of security to shield material from cyber assaults as well as hackers. It is time-consuming to locate files, yet a DMS can recover documents by seeking a key phrase or phrase. DMS can also index groups within a file or folder and allow an also smoother search.
Users can access records from various resources from multiple areas. DMSes likewise offer variation control, which is required for individuals to recover older variations of records.
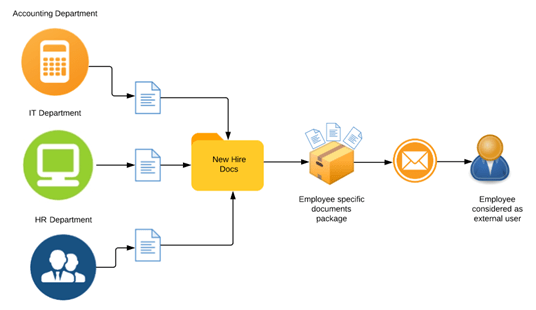
The Only Guide to Copier Solutions In Santa Fe Springs Ca
What design template to utilize for every sort of record. What metadata to offer each type of paper. Where to save a file at each stage of its life process. How to manage accessibility to a record at each stage of its life process. Just how to relocate papers within the organization as employee add to the files' production, testimonial, authorization, magazine, and disposition.
Share, Point Server consists of the exact same functions and additionally adds: What policies to use to papers to ensure that document-related actions are audited, documents are maintained or disposed of suitably, as well as web content that is very important to the organization is safeguarded. Exactly how to manage records as business records, which need to be retained according to legal demands as well as company standards.
Share, Point Server uses a series of features to aid organize and save Managed Voice Services in Santa Fe Springs CA documents, from specialized sites to freely structured record collections for quick paper creation and cooperation. Within a collection, you can in addition arrange material into folders and also subfolders. It could be essential to relocate or duplicate a document from one website or collection to one more at various phases of its life cycle.
Network Services Provider In Santa Fe Springs Ca Can Be Fun For Anyone
Keep in mind Plans are not readily available in Share, Factor Structure 2013 - Network Services Provider in Santa Fe Springs CA.
In the life scientific researches sector, paper control signifies the processes and also strategies made use of for taking care of the several different records that action within the company, amongst capitalists and also sponsors, as well as regulatory agencies throughout the lifecycle of the item. Documentation is vital to show the safety and security as well as effectiveness of your company's items such as clinical gadgets as well as drugs.
With documentation, you are efficiently connecting what has to be done, when it needs to be done, as well as just how it has actually to be done. These are the subjects we will certainly cover: Document control can be specified as a series of practices that ensure that records are developed, examined, dispersed, and disposed of in an arranged and verifiable fashion.
The Only Guide for Office Technology Supplier In Santa Fe Springs Ca
While these terms are carefully additional hints related, they are not compatible. Record monitoring indicates the systems as well as processes your organization has established for maintaining and also handling documentation. It is the storage space, place, tracking, updating, and also sharing of documents. Let us take a look at some examples from the life scientific researches sector to obtain a far better understanding of record control.
As your company begins conforming with one requirement, you will certainly also be preparing for the other requirement. The company should recognize the most suitable person(s) for analysis of all procedures concerning relevant documents, All record control authorizations should include signatures of approvers and the date, All appropriate factors of use must wikipedia reference have the current version of the paper, Modifications, if called for, must be evaluated as well as authorized by the very same individual(s) that were associated with the original testimonial as well as approval, Authorized modifications in papers have to be connected to all appropriate individuals The most up to date clinical gadget regulation applicable to the European Union has several points concerning record control.
This is a collection of quality laws as well as standards to ensure that products produced by the life sciences market are secure, appropriate for their pictured usage, and follow all quality methods throughout their manufacture, control, storage space, and also a phrase permanently (x-variable relying on application- for example, Medical; Lab; Manufacturing; Storage; Evaluation.) Method.